Dalton's Law of Partial Pressure
Understand Daltons Law of Partial Pressures. Hydrogen Oxygen Water 2g 16g 18g.

Daltons Law Of Partial Pressures Easy Science Dalton S Law Easy Science Organic Chemistry Study
The total pressure of the gas mixture is the sum of the partial pressure of the component gases.

. At high pressure the volume occupied by a gas becomes significant when compared to the free space between particles. To Learn expressions on Daltons law of partial pressure Examples Videos with FAQs. The oxygen is usually the only metabolically active component unless the gas is an anaesthetic mixture.
The partial pressure is the pressure that each gas would exert if it alone occupied the volume of the mixture at the same temperature. Visit BYJUS for more content. And from the alveolar ducts to the alveoli the tract is lined with simple squamous epithelium.
In 1803 he revealed the concept of Daltons Law of Partial Pressures. The essential component for any breathing gas is a partial pressure of oxygen of between roughly 016 and 160 bar at the ambient pressure. Developed by chemist and physicist John Dalton who first advanced the concept of chemical elements being made up of atoms 9 X Research source Daltons Law states that the total pressure of a gas mixture is the sum of the pressures of each of the gases in the mixture.
Definition of partial pressure and using Daltons law of partial pressures. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 15 atm on the walls of its container. Definition of partial pressure and using Daltons law of partial pressures.
Also in the 1800s he was the first scientist to explain the behavior of. Triple Point - Triple points for common substances. This law was proposed by Dalton in 1803 and is also known as Daltons Law or Daltons Law of multiple proportions.
Some of the oxygen in the breathing gas is consumed by the metabolic processes and the inert components are unchanged and serve. Charles Law Boyles Law and Avogadros Law all of which will later combine into the General Gas Equation and Ideal Gas Law. The histology along the respiratory tract changes from the trachea to the tertiary bronchi the tract is lined with ciliated pseudostratified columnar epithelium smooth muscle and cartilage rings.
The bronchioles are lined with cuboidal epithelium. He also developed methods to calculate atomic weights and structures and formulated the law of partial pressures. Daltons law of partial pressures states that the pressure of a mixture of gases simply is the sum of the partial pressures of the individual components.
P tot the total pressure. The Ideal Gas Law - The relationship between volume pressure temperature and quantity of a gas including definition of gas density. It is only an approximation for real gases.
Hydrogen Oxygen Hydrogen Peroxide. If the partial pressure of hydrogen is 1 atm find the mole fraction of oxygen in the mixture. The pressure of any gas within the container is called its partial pressure.
Daltons law is as. Created in the early 17th century the gas laws have been around to assist scientists in finding volumes amount pressures and temperature when coming to matters of gas. Daltons law is an ideal gas law.
Where P is the mole fractions of the components. Limitations of Raoults Law. Total and Partial Pressure - Daltons Law of Partial Pressures - How to calculate total pressure and partial pressures for gas mixtures from Ideal Gas Law.
Daltons law the statement that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the individual component gases. PA XA PB XB XAPA XBPB. The limitations of Raoults Law are as follows.
P p 1 p 2 p 3 p n n i1 p i. P tot P i P 1 P 2 P 3. This empirical relation was stated by the English chemist John Dalton in 1801.
This new pressure is the partial pressure of each A and B and is given by Raoults law and depends on the concentration of each component in the liquid phase. Avogadros law states that equal volumes of all gases at the same temperature and pressure have the same number of molecules. Although a schoolteacher a meteorologist and an expert on color blindness John Dalton is best known for his pioneering theory of atomism.
The deviation from the law increases with increasing pressure. If youre seeing this message it means were having trouble loading external resources on our website. V 1 n 1 V 2 n 2.
Partial Pressure- Partial Pressure is defined as a container filled with more than one gas each gas exerts pressure. Back to top. Since equal volumes have equal number of molecules this is the same as being inversely proportional to the root of the molecular weight.
The gases present in the container are chemically inert. Solved Examples on Daltons Law of Partial Pressure Example 1. Deviations From Daltons Law.
Combined with Avogadros law ie. For example Hydrogen combines with oxygen to form two compounds one water and another hydrogen peroxide. The gas laws consist of three primary laws.
The term partial pressure is used when we have a mixture of two or several gases in the same volume and it expresses the pressure that is caused by each of the induvidual gases in the mixture. P i the. Early Life dalton1-profilejpg John Dalton FRS engraved by William Henry Worthington after an 1814 painting by William.
Daltons law of partial pressures states that the pressure of a mixture of gases simply is the sum of the partial pressures of the individual components.

15 12 6 Dalton S Law Of Partial Pressure In Mixtures Of Gases Each Component Gas Behaves Independently Of The Other S In 2022 Ideal Gas Law Molecular Physics Formulas

Dalton S Law Of Partial Pressures Explained
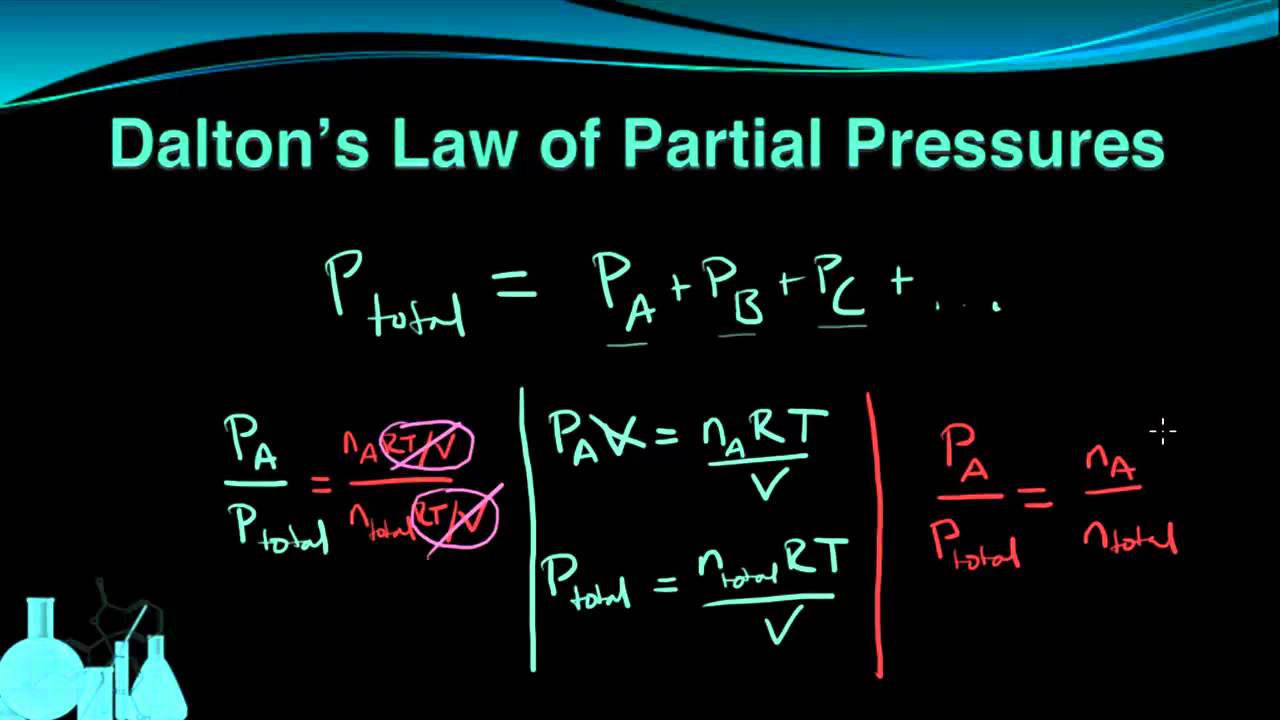
Chemistry 7 6 Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton

Dalton S Law Of Partial Pressure Dalton S Law 11th Chemistry Chemistry
Comments
Post a Comment